The Timeless Soxhlet Method: From Milk Fat to Modern Innovation

Today, we're taking a trip back in time to talk about a true workhorse in chemistry: the Soxhlet method. It's a method that's been around for ages, and it's a perfect example of how a simple, brilliant idea can stand the test of time.
The Man Behind the Method
The story of the Soxhlet method begins with Franz von Soxhlet, a German agricultural chemist. Much of his research career was spent investigating the properties of milk. He was the first to describe lactose and to separate the major milk proteins: casein, albumin, lactoprotein and globulin. He was the first to suggest that the recently invented process of pasteurisation be applied to milk, and later he developed a simple sterilisation (pasteurisation) device which could be used on babies’ bottles.
In 1879, Soxhlet designed a piece of laboratory glassware to determine the fat content in milk. He published a paper, “Die gewichtsanalytische Bestimmung des Milchfettes”, in Dingler’s Polytechnisches Journal, in which he described this new Soxhlet extractor.
It caught on rapidly and became widely used across chemistry, biochemistry, the food, plastics and oil industries – anywhere which required exhaustive extractions. What he created was a continuous extraction apparatus that revolutionized the process. Before him, extracting a substance from a solid material was a tedious, manual process that involved repeatedly washing the solid with fresh solvent. Soxhlet's clever design automated this, making it far more efficient. His invention essentially created a closed loop where a solvent would evaporate, condense, drip down onto a sample, and then siphon back into the boiling flask, carrying the extracted compound with it. This allowed for a highly effective and consistent extraction.
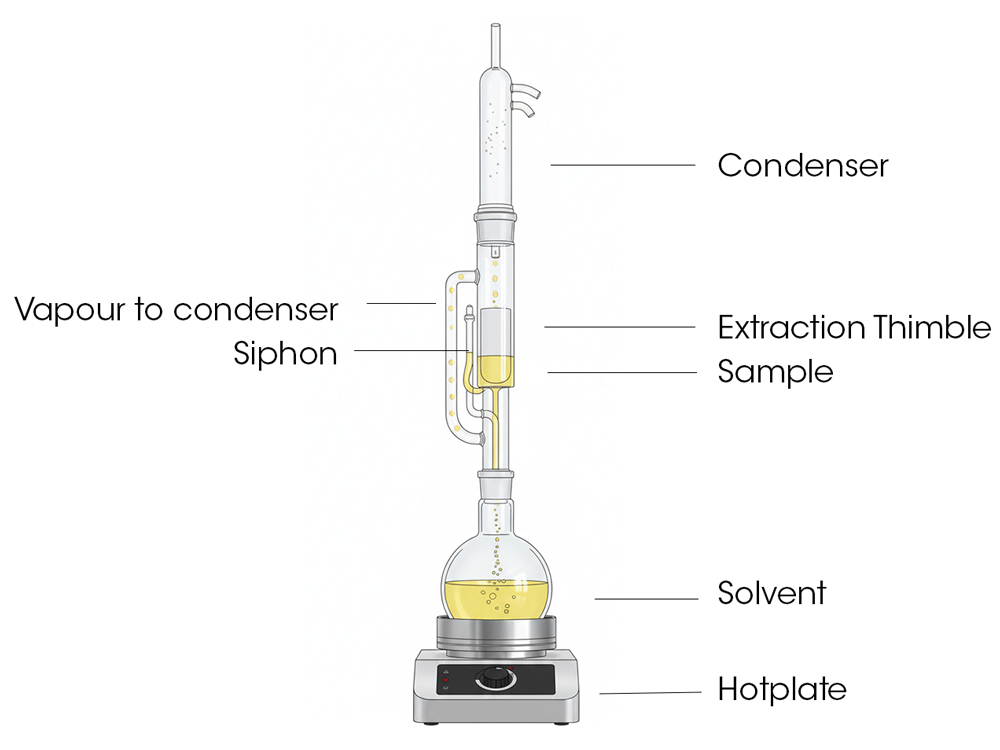
Soxhlet Extractor
A Century of Development
While the fundamental design of the Soxhlet apparatus has remained largely unchanged, its application has grown immensely over the last century. From its humble beginnings in milk analysis, it became a standard method for extracting lipids, natural products, and even certain pollutants from a wide variety of solid samples. Its key advantage is that it allows for a prolonged and thorough extraction with a relatively small volume of solvent. The development during this period hasn't been about inventing a completely new method, but rather about refining the process.
Early developments largely focused on optimizing the traditional Soxhlet. Researchers experimented with different sizes of glassware, improved heating methods like water baths and heating mantles that offered more controlled temperatures than open flames, and better ways to recover and recycle solvents. These incremental improvements made the method more reliable and safer to operate in a general laboratory setting.
As demand for faster analysis grew, variations emerged that aimed to speed up the process. Some designs incorporated features for increased heat transfer to the sample, while others looked at reducing the cycle time of the solvent. The challenge was always to balance the thoroughness of a complete extraction with the desire for quicker results, without compromising the integrity of the extracted compound. This led to a continuous refinement of apparatus designs, materials and operational procedures. Many methods such as Randall, Twisselmann, Goldfish etc. was developed during these years.
The method itself, though, remains: a condenser, an extraction chamber, and a flask where the solvent is boiled and circulated. It's fascinating how such a simple principle has stayed relevant, even though we all know the world moves forward in small steps.
Challenges and Choices with Solvents
Choosing the right solvent is perhaps the most critical part of the Soxhlet method, and it's where we face some interesting challenges, even today. The choice depends on the properties of the compound we're trying to extract. The solvent needs to be able to dissolve the target compound well, but not the rest of the solid material.
- For non-polar compounds like fats and oils, petroleum ether or hexane are often used. These are great because they dissolve fats readily and have a low boiling point, making them easy to remove from the final extract. The main risk here is their high flammability. They require careful handling and good ventilation.
- For compounds with some polarity, like certain alkaloids or pigments, solvents such as ethanol or methanol are a better choice. Ethanol, in particular, is often preferred for food-related extractions because it's considered safer than many other solvents. However, these alcohols can also extract water-soluble compounds, which may not be desirable.
- And then there's diethyl ether, a very volatile and flammable solvent often used for lipid extraction. Its low boiling point is an advantage, but its volatility and the risk of forming explosive peroxides make it one of the more dangerous solvents to work with.
Each solvent brings its own set of advantages and risks, and the chemist must carefully weigh these when deciding on the best one for their specific application. The Soxhlet method, while simple in principle, truly relies on this careful consideration of chemistry to be successful and safe. The risks with these solvents are not to be underestimated, and it's important to approach them with respect. Overall, it's always recommended to work in a fume hood, use gloves, and avoid unnecessary contact to minimize the dangers. It's a reminder that even simple methods require vigilance.
Modern Innovations for the Extraction
While the traditional Soxhlet method is a marvel of simplicity, modern laboratories demand higher levels of automation, safety, and efficiency. This is where instruments like the OPSIS LiquidLINE SoxROC come into play. It takes the core principle of hot solvent extraction and elevates it with contemporary technology.

OPSIS LiquidLINE SoxROC
In a traditional setup, a chemist must manage flammable solvents, hot glassware and the manual transfer of samples, all of which pose significant risks. The SoxROC, however, addresses these challenges head-on. It's a closed system that automates the entire extraction process, from boiling and rinsing to solvent recovery. This means less hands-on time for the user and a drastic reduction in the chance of spills, burns or exposure to hazardous fumes. The instrument has a protection shield and features like closed addition of solvents and an easy-to-remove recovery tank, which are all designed with the operator's safety in mind.
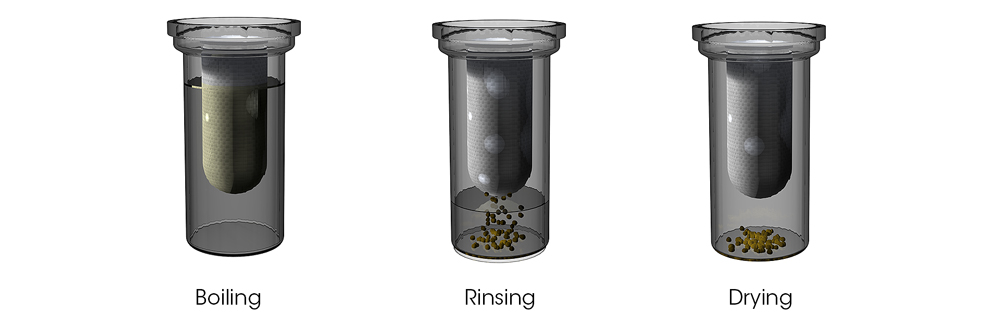
SoxROC principle with automatic boiling, rinsing, and drying
The SoxROC also has built-in safety features, such as ATEX-classified components for parts that are in contact with solvents, which means they are designed to be safe for use in potentially explosive atmospheres. It also has a pressurized electronics cabinet, ensuring that no solvent vapours can enter and cause an electrical spark.

The SoxROC has a separate pressurized electronics cabinet
By using this type of automated system, a laboratory can greatly enhance its safety protocols and make the process of hot solvent extraction much more secure for its staff. It's an example of how OPSIS LiquidLINE works to improve traditional methods with innovation and careful engineering, with a focus on the well-being of the user.
Please contact us to today to learn more about our development with extraction instruments.
SoxROC webinar
You can access a link to a previously held SoxROC webinar here >>
Description of the webinar: We dive deeper into the solvent extraction method and showcase a live demonstration of our SoxROC extraction unit in the lab.
Author: Olle Lundström, OPSIS LiquidLINE